TANNERS-CHEM-BLOGS
Monday, April 22, 2013
Monday, March 4, 2013
3 Labs Temperature... Volume... Pressure
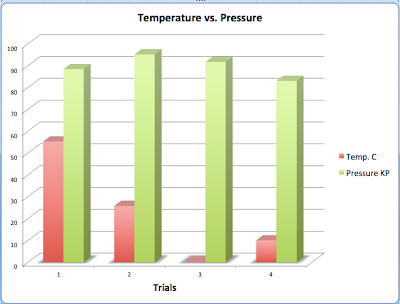
This lab was all about the topics of temperature, volume, and pressure. We measured all three of these in suringes. My group figured out some pretty basic knowledge. If the temperature of the test tube goes up the pressure will go up. This is called Guy-Lussac's law. If the temperature goes down the pressure will go down, which is called Charles law. We also found out if the pressure goes up the volume of the suringe will go down. The graphs below are the data we discovered in the lab over the days.
Thursday, February 7, 2013
Monday, January 21, 2013
Mole Calculation
Over the past two weeks we have been working on mole calculations. This is very different because mole is a very huge number. At first I really didn't understand it, but as we got more practice I figured it out. All the sheets below are a few of the worksheets that we have been doing to help improve our mole calculation skills. The mole identity lab was very interesting because we add to find out what time of metal it was, just by using the mole calculations. We have done many problems over the last couple weeks to help everyone in class understand how to do problems in moles.
Monday, December 17, 2012
Naming Compounds & Formulas
In class we learned how to name compounds and formulas. At first I had trouble learning how to name and write out the formulas. We took a test on this I didn't do so good at first but the second time I fixed it up and got the hang of it. We did this lab called " If your Cat took chemistry, would she eat this stuff?" This lab was to help us with naming formulas and compounds. We also had these cheat sheets that help me learn how to write the formulas out and it also had the names of the compounds, so it was big help.
These are the cheat sheets that helped me solve all the problems. They help a lot. ====>
This is the test I had trouble on at first but after a while I got the hang of it. I fixed my mistakes on this one I only missed each problem by a hair!
Thursday, December 13, 2012
TYPES OF REACTIONS
This lab we did seven mini labs. This was very interesting because each reaction reacted different from one another. The purpose of this laboratory activity is to discover the characteristics of different types of reactions.
BALANCED EQUATIONS:
Number 1: HC1+Mg ==> MgCl2+H2
Number 2: 2Mg+O2 ====>2MgO+ Energy (Combustion Reaction)
Number 3: Cu+O2 ===>2CuO+ Energy
2
Number 4: (NH4)2 CO3 ===> 2NH3 + CO2 + HO2 (Decomposition Reaction)
Number 5: 2H2O2====> 2H2O2+O
Number 6: Pb(NO)3+ 2Kl ==> Pbl2+ KnO3
Number 7: CuCO3 ===> CuO+O2 (Decomposition Reaction)
These are the balanced equations and what kind of reaction each little lab was. The papers above explain what procedures we did on each mini lab. This lab we learned a lot on reactions and we learned how to balance them this lab was fun because we got to see many different kinds of reactions in one lab!!
Number 5: 2H2O2====> 2H2O2+O
Number 6: Pb(NO)3+ 2Kl ==> Pbl2+ KnO3
Number 7: CuCO3 ===> CuO+O2 (Decomposition Reaction)
These are the balanced equations and what kind of reaction each little lab was. The papers above explain what procedures we did on each mini lab. This lab we learned a lot on reactions and we learned how to balance them this lab was fun because we got to see many different kinds of reactions in one lab!!
Wednesday, December 5, 2012
PERIODIC TABLE
The periodic table is a very important thing we use as a tool in Chemistry class. The periodic table is table of elements which are arranged by order of atomic number. The standard form of the table includes periods which are horizontal on the periodic table. Groups are also important in the periodic table they are the vertical columns on the periodic table. Elements in groups have some similar properties to each other. There is no one single or best structure for the periodic table but by whatever consensus there is, the form used here is very useful. The periodic table is such a useful tool in chemistry that we have used for hundreds of years to solve problems about our universe. Many chemists have put the time in to form this periodic table and it is still being added to now days. On the periodic table it consists of metals and non-metals. There are also groups in the metals which are named, alkali metals, alkaline earth metals, lanthanoids, actinoids, transition metals, and post-transtion metals. Each of these groups consists of metals on the periodic table and they have similar chemical properties which why they are classified. The non-metals are classified in groups called, metalloids, other non-metals, halogens, and noble gases. Each element has its own atomic number, the atomic number tells how many protons are in an element. Elements in the periodic table also have mass numbers, which tell how many neutrons and protons are in the element. There periodic table is used a lot in our chemistry class and is a great tool to help with many problems.
1: Atomic Number
Hydrogen: NAME
H: Symbol
1.008: Mass Number
We used this website called ptable.com. This was a very good website to help with the periodic table. We did this worksheet where we had to find the boiling point, melting point, density, atomic radius, electronegatvity, and number of isotopes. This website gave us all this information of elements on the periodic table.
1: Atomic Number
Hydrogen: NAME
H: Symbol
1.008: Mass Number
We used this website called ptable.com. This was a very good website to help with the periodic table. We did this worksheet where we had to find the boiling point, melting point, density, atomic radius, electronegatvity, and number of isotopes. This website gave us all this information of elements on the periodic table.
Subscribe to:
Comments (Atom)